Laws of Thermodynamics
Laws of Thermodynamics
Thermodynamics is related to science which narrates the relations between heat and mechanical work. It is the branch of Physics which is based on four laws. It exchanges the various forms of energy. Thermodynamics system includes various products like Heat Conduction Apparatus, Air Humidity Measurement Trainer and Heat Conduction Apparatus etc. All the systems work based on four laws. We have described here under the entire four laws.
Four Laws of Thermodynamics:
- Zeroth law of thermodynamics
- First law of thermodynamics
- Second law of thermodynamics
- Third law of thermodynamics
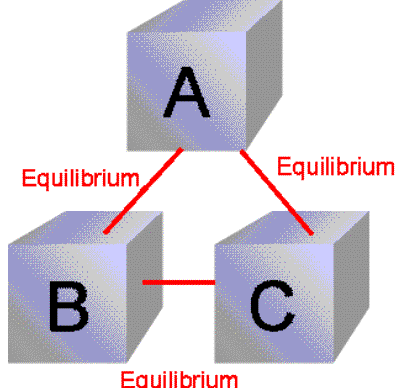
Zeroth law of thermodynamics: According to this law if two systems named as A and B is in equilibrium with the third system named as C then A and C are also in thermal equilibrium.
First Law of thermodynamics:
We can explain this law in various ways.
Second Law:
Second law concludes that the total sum of all the entropies is enhanced in every natural thermodynamic process that means if there will be same temperature then no work will be done.
Third Law:
This law states that the entropy of a crystal will be zero when the temperature of the crystal is absolute zero. Entropy is related to purity which is at zero. Third law supports the use of first two laws. It is used in Ultra-low temperature chemistry and physics. It refers to the stat of “absolute zero”.
These were the four laws of thermodynamics in which it works.

